EHDS in focus: stakeholders meet at TEHDAS2 forum during the European Health Data and Innovation Summit
European health professionals convened to discuss the future of the European Health Data Space (EHDS) and digital health, following the recent adoption of the EHDS regulation. The forum also coincided with the launch of the first TEHDAS2 public consultations.
On 30 January, TEHDAS2, the second joint action Towards the European Health Data Space, hosted its first stakeholder forum in Warsaw, Poland, and online as part of the European Health Data and Innovation Summit. The event drew nearly 350 in-person participants and attracted over 2,000 online views. Discussions centered on the future of the EHDS and what is needed to ensure the regulation’s success.
The larger conference was organised in collaboration with the Polish Chamber of Physicians and Dentists (NIL), EOSC4Cancer, Polish Ministry of Health and EIT Health.
Opening remarks highlight the potential of high quality data
With Poland presiding over the EU Presidency, the event was opened by Artur Drobniak, President of the Regional Medical Chamber in Warsaw, followed by Urszula Demkow from the Polish Ministry of Health, who outlined Poland’s health priorities during its EU Presidency.
Their presentations set the stage for the event, emphasising the transformative potential of high-quality data and digital innovation in shaping the future of healthcare through improved care quality, reduced costs and enhanced research and innovation. “Data is an essential resource for economic growth, competitiveness, innovation and a healthy society,” said Demkow.
EHDS: a huge opportunity for member states
The forum continued with a dialogue between Joni Komulainen from the Finnish Ministry of Social Affairs and Health and Emilie Passemard from the French Ministry of Health, who shared their countries’ respective approaches to EHDS implementation. While Finland has focused on building technical infrastructure and harmonising healthcare systems, France has prioritised citizen engagement to ensure data sovereignty, transparency and public trust. Both stressed the importance of EU financial support and stakeholder collaboration in ensuring the EHDS’s success. Komulainen also highlighted the importance of TEHDAS2 in supporting the impelementation.
In talking about the broader impact, Komulainen stressed that the EHDS benefits not just specific stakeholders like researchers, industry and AI developers, but everyone, emphasising how the EHDS will lead to better healthcare, innovation and governance.
The EHDS is a collective project, and its success depends on your active participation.
Fulvia Raffaelli, DG SANTE, European Commission
The clock has started ticking for implementation
With just four years to make the EHDS fully operational, the focus is now on meeting this ambitious timeline. Discussions at the forum underscored that, while the EHDS presents a significant opportunity, its success will depend on careful execution and close collaboration among all stakeholders, including researchers, industry professionals and citizens.
As Fulvia Raffaelli from the European Commission highlighted in her virtual address, “The European Commission cannot do this alone. The EHDS is a collective project, and its success depends on your active participation.” She encouraged everyone to take part in the open TEHDAS2 public consultations supporting the EHDS implementation.
“You can influence our work” – TEHDAS2 paves the way for EHDS implementation
“We’re creating guidelines and technical specifications – practical documents that help the implementation,” explained Markus Kalliola, TEHDAS2 Co-ordinator and Programme Director at Sitra, describing the joint action’s outputs. “We’re doing these for data users, data holders and health data access bodies.”
The joint action, involving 29 European countries, aims to prepare member states and stakeholders for EHDS implementation by improving coordination and reducing fragmentation.
Kalliola outlined the five key steps from a user journey perspective in the secondary use of health data within the EHDS: data discovery, data access, data preparation, data use and study finalisation. “For each of these steps, TEHDAS2 is providing guidelines,” he added. Additional documents under development include, for example, a guideline on fees, a guideline on informing citizens about data use and a technical specification on secure processing environments.
Kalliola strongly encouraged everyone to provide feedback on the published documents. For more information on the guidelines and public consultation surveys, visit our web page.
First TEHDAS2 guidelines are open for public input
TEHDAS2 partners Nienke Schutte, Ann Gustaffson, Dzenka Dudová, Pia Brinkmann and Marianne Benderra guided the audience through and provided an overview of the first four documents available for public consultation.
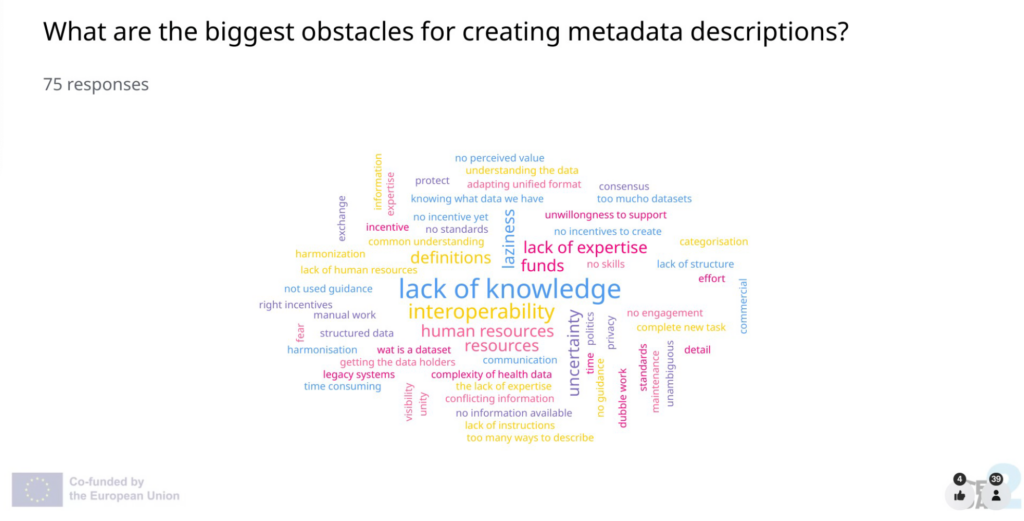
Nienke Schutte (Sciensano) introduced the TEHDAS2 draft guideline on data description, which helps health data holders fulfill their duties under the EHDS.
Schutte emphasised that the guideline’s greatest value lies in improving dataset discoverability, ultimately benefitting health data users. “A single dataset description standard is really needed to enhance data discovery, to make sure that all datasets are described consistently, and organised and shared in the same manner,” she explained. This standard will improve interoperability and help users locate high-quality data more efficiently. “In the end, the biggest value will be that the health data user can benefit from enhanced dataset discovery throughout the EHDS.”
Ann Gustaffson (Swedish eHealth Agency) introduced the TEHDAS2 technical specification on the national metadata catalogue, which will ensure interoperability between national and European health data catalogues.
Gustaffson explained the four key capabilities of the catalogue: input (submission of detailed dataset descriptions by data holders), management (storage, validation and maintenance by health data access , bodies), output (exporting dataset descriptions in HealthDCAT-AP format to the national contact point, and from there to the HealthData@EU Central Platform) and access (easy findability and access of datasets by data users). She emphasised that the specification is deliberately drafted at a high level to provide flexibility for member states in developing more detailed steering documents.
Zdenka Dudová (Masaryk University) spoke about the user perspective on the data access application and request process, highlighting the value of the TEHDAS2 guideline in providing clear instructions to data applicants on regulatory compliance and how to become a data user.
“EHDS is giving transparent and standardised procedures, and it’s great that we could summarise it in this guideline,” she noted. The document explains the Healthdata@EU infrastructure from the applicant’s perspective and how to use the form, “which is the only one in the whole Europe.” She emphasised that, ultimately, “the broader public will benefit from these advancements in healthcare and policy driven by improved access.”
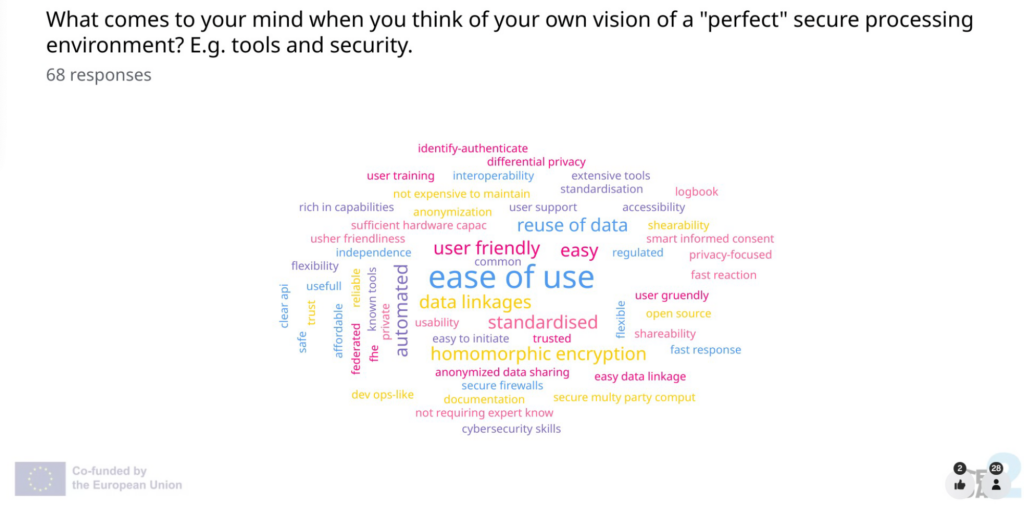
Pia Brinkmann (Germany’s Federal Institute for Drugs and Medical Devices) and Marianne Benderra (French Health Data Hub) walked the audience through the fourth TEHDAS2 draft guideline, which is designed to help health data users navigate secure processing environments (SPEs). They emphasised that the goal is to ensure that all users clearly understand what processing personal health data involves and the necessary steps to follow.
Designated commentators provided additional insights after each guideline presentation. For example, Evangelos Markatos (University of Crete) suggested including pre-filled application examples, a list of common pitfalls and data minimisation examples to help applicants avoid mistakes and improve approval rates. The TEHDAS2 partners will carefully consider all feedback received.
Speakers encouraged stakeholders to take part in the ongoing public consultation, with the final versions of the documents expected in summer 2025.
Can EHDS be successful enough for Europe to prove it can innovate – not just regulate?
Artur Olesch
Closing panel: EHDS and the future of healthcare innovation
“Can EHDS be successful enough for Europe to prove it can innovate – not just regulate?” asked Artur Olesch, Digital Health Journalist and moderator of the panel debate “Future Outlook of EHDS and Digital Health in Europe.”
In this final panel of the stakeholder forum, experts highlighted the EHDS’s potential to transform healthcare through AI-driven diagnostics, cross-border data sharing and medical research. They emphasised the need for interoperability, ethical data use, skills development and continuous stakeholder collaboration. Ultimately, technological readiness, regulatory clarity and – most importantly – building trust will be central to determining the success of the EHDS.
The panelists included Dr Salvador Capella Gutierrez (EOSC4Cancer), Artur Drobniak (NIL), Mélodie Bernaux (European Commission), Myriam Fernández (AWS) and Jean-Marc Bourez (EIT Health).
EOSC4Cancer and TEHDAS2 workshop: Bringing EHDS into Cancer Research
As part of the stakeholder forum, TEHDAS2 co-organised a workshop with EOSC4Cancer titled Bringing EHDS into Cancer Research. In this session, experts discussed how to better integrate the EHDS with cancer research, exploring both opportunities and possible challenges.
One of the key takeaways was the potential for cancer data nodes to serve as health data intermediaries, highlighting the need for synergies in governance, technical infrastructure and personnel. Insights from the workshop will inform EOSC4Cancer’s Roadmap for a Cancer Data Space. You can read more on the joint workshop from the website of EOSC4Cancer.
Partners wrap up successful event in Poland with general assembly
A day after the stakeholder forum, TEHDAS2 project participants gathered at the Polish Ministry of Health and online for the project’s second general assembly. The meeting provided an opportunity to reflect on progress so far and discuss the next steps.
Project co-ordinator Markus Kalliola gave an overview of the joint action, emphasising the importance of continued collaboration to reach project goals. Work package leaders shared updates on their progress, while both formal and informal discussions sparked new ideas for advancing the project.
To further align the project’s activities with recent European developments, representatives from the European Commission’s DG SANTE outlined the Central Services that will be an integral part of the EHDS and the framework for the secondary use of health data. Additionally, an external advisory board – comprising experts in health policy, digital health and data – offered recommendations to help steer the project forward.
By bringing consortium members from across Europe together, the assembly strengthened collaboration and re-energised collective efforts.
Revisit the recording: Broadcast from the Main Stage
Watch a summary of the TEHDAS2 stakeholder forum/European Health Data and Innovation Summit
The TEHDAS2 public consultation on the draft EHDS implementation guidelines is open until 28 February. You can review the documents and submit your feedback via the surveys available on the TEHDAS2 website. The consultation period will close on 28 February 2025.
The next TEHDAS2 stakeholder forum will take place in Denmark in October 2025.
To stay informed about the next rounds of public consultations and stakeholder forums, we encourage you to subscribe to our newsletter via tehdas.eu. You can also follow us on LinkedIn for regular updates.
Share away!
